Pubblication
Structural insight into the stabilization 1 of microtubules by taxanes
Mar 07, 2023 | News
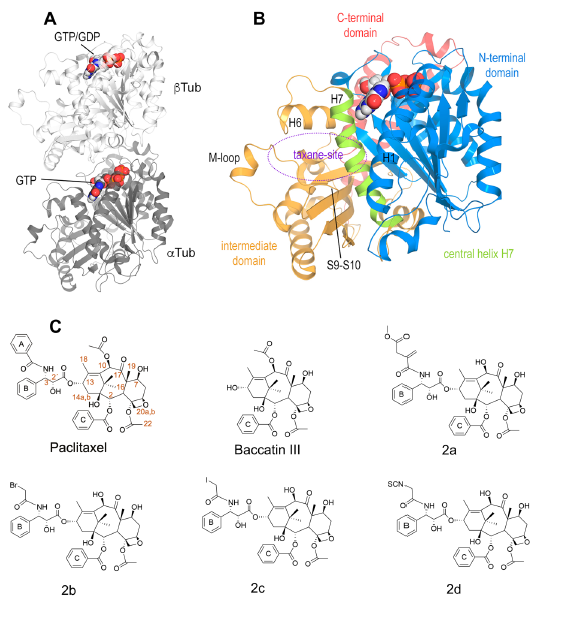
Paclitaxel (Taxol®) is a taxane and a first-line chemotherapeutic drug that stabilizes microtubules. While the interaction of paclitaxel with microtubules is well described, the current lack of high-resolution structural information on a tubulin-taxane complex precludes a comprehensive description of the binding determinants that affect the drug’s mechanism of action. Here, we solved the crystal structure of the core baccatin III moiety of paclitaxel lacking the C13 side chain in complex with tubulin at 1.9 Å resolution. Based on this information, we engineered two tailor-made taxanes with modified C13 side chains, solved their crystal structures in complex with tubulin, and analyzed their effects along with those of paclitaxel, docetaxel, and baccatin III on the microtubule lattice by X-ray fiber diffraction. We then compared high-resolution structures of ligand-bound tubulin and microtubule complexes with apo forms and used molecular dynamics simulations to understand the consequences of taxane binding to tubulin as well as to simplified protofilament and microtubule-lattice models. Our combined approach sheds light on three mechanistic questions. Firstly, taxanes bind better to microtubules as compared to unassembled tubulin due to a dual structural mechanism: Tubulin assembly is linked to a conformational reorganization of the bM loop, which otherwise occludes ligand access to the taxane site, while the bulky C13 side chains preferentially recognize
the microtubule-assembled over the unassembled conformational state of tubulin. Second, the occupancy of the taxane site by a ligand has no influence on the straightness of tubulin protofilaments. Finally, binding of the taxane core to the taxane site displaces the S9-S10 loop of b-tubulin resulting in microtubule expansion. Our results provide
detailed new insights into the mechanism of microtubule-stabilization by taxanes.
https://www.tubintrain.eu/wp-content/uploads/2023/03/2021.07.20.453061v2.full_-1.pdf
Written by tubinAD
